
Insights+: The US FDA New Drug Approvals in May 2023
Shots:
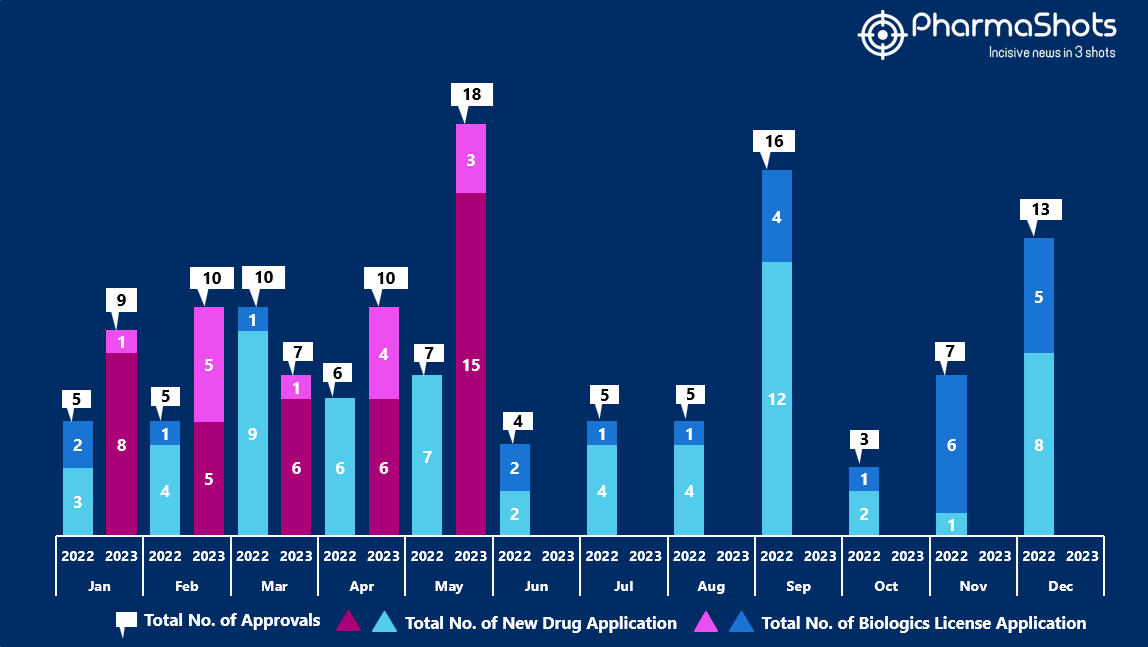
- The US FDA approved 15 NDAs and 3 BLA in May 2023, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 54 novel products in 2023
- In May 2023, the major highlights drugs were, Ayvakit approval for Indolent Systemic Mastocytosis, Rinvoq for Active Crohn's Disease
- PharmaShots has compiled a list of a total of 18 new drugs approved by the US FDA in May 2023
Uzedy
Active ingredient: risperidone Approved: May 01, 2023
Company: Teva and MedinCell Disease: Schizophrenia
- The US FDA has approved Uzedy (risperidone) extended-release injectable suspension for the treatment of adults with schizophrenia
- The approval was based on the results from the 2 P-III study (RISE) & (SHINE) evaluating the efficacy of risperidone extended-release injectable suspension for SC use in 544 & 336 patients aged 13-65yrs. with schizophrenia. The results showed that the patients treated with Uzedy achieved a ~80% reduction in risk of schizophrenia relapse over PBO
- Uzedy, the first SC, long-acting formulation of risperidone that has been developed using SteadyTeq (copolymer technology) to MedinCell that controls the steady release of risperidone
Varenzin-CA1
Active ingredient: molidustat sodium Approved: May 02, 2023
Company: Elanco Animal Health Disease: Chronic Kidney Disease
- The US FDA has conditionally approved the first drug, Varenzin-CA1 for the management of nonregenerative anemia in cats with CKD. The US FDA was granted the expanded conditional approval authority in the Animal Drug User Fee Act of 2018 with the program planned to sunset in 2028
- The efficacy of molidustat oral suspension had been established based on the results of a study conducted in 2 phases in 23 cats aged 4-17yrs. from various breeds or breed mixes. Varenzin-CA1 can increase the production of erythropoietin in the kidney & stimulates the bone marrow to produce more red blood cells
- Varenzin-CA1 is in the form of a liquid, administered orally, qd for ~28 days & can be repeated as needed after a minimum of 7-day pause. The drug is only available by prescription from a licensed veterinarian
3. Zoetis’ Librela (bedinvetmab) Receives the US FDA’s Approval to Control Osteoarthritis Pain in Dogs
Librela
Active ingredient: bedinvetmab Approved: May 08, 2023
Company: Zoetis Disease: Osteoarthritis Pain
- The US FDA has approved the first anti-NGF mAb treatment, Librela (qm) for the control of pain associated with OA in dogs
- The results from 2 field studies showed a reduction in OA over PBO and reduce pain. Acc. to studies, Librela improves people's mobility & overall QoL while Librela's effectiveness might not become apparent until after 2nd dose, dogs may also experience a reduction in pain as early as 7days after 1st dose
- Additionally, dogs treated with bedinvetmab experienced lasting OA pain relief over the course of the study with monthly inj. in a continuation study. The therapy is expected to be available in the US in late 2023 & was approved for use in Canada, Brazil, Australia, New Zealand, Japan & other markets
Elfabrio
Active ingredient: pegunigalsidase alfa Approved: May 10, 2023
Company: Chiesi Global Rare Diseases Disease: Fabry Disease
- The US FDA has approved Elfabrio for the treatment of adults with Fabry disease. The approval was based on a comprehensive clinical development program evaluating Elfabrio in 140+ patients (ERT-naïve & ERT-experienced patients) with up to 7.5 years of follow-up treatment incl. a head-to-head trial
- The trials met its 1EPs & showed that pegunigalsidase alfa was non-inferior to agalsidase beta in controlling eGFR decline, the estimated mean eGFR slope was -2.4 vs -2.3mL/min/1.73 m2/yr., estimated treatment difference was -0.1mL/min/1.73 m2/yr. & was well-tolerated
- Elfabrio is supplied as a preservative-free solution in a single-dose vial while each vial contains 20mg/10mL of pegunigalsidase alfa-iwxj. Treatment is administered by IV infusion, q2w
Farxiga
Active ingredient: dapagliflozin Approved: May 10, 2023
Company: AstraZeneca Disease: Heart Failure
- The US FDA has approved Farxiga to reduce the risk of CV death, hospitalization for heart failure & urgent HF visits in adults with HF
- The approval was based on the P-III trial (DELIVER) evaluating dapagliflozin (administered as qd in addition to background therapy) vs PBO in 6263 randomized patients with HF with LVEF ≥40% with/out T2D. The results showed an early reduction in the primary composite EPs of CV death or worsening HF in patients with HF with HFmrEF or HFpEF
- The results were published in the NEJM. Farxiga was approved in the US for adults with HFrEF & was also approved for T2D, HFrEF, and CKD in 100+ countries globally incl. the US, the EU, China, and Japan
Rexulti
Active ingredient: brexpiprazole Approved: May 12, 2023
Company: Otsuka and Lundbeck Disease: Alzheimer’s Disease
- The US FDA has approved the sNDA of Rexulti for agitation associated with dementia due to AD. The sNDA was based on 2 P-III 12wk. fixed-dose studies (Study 331-12-283) and (Study 331-14-213) evaluating brexpiprazole vs PBO
- The results from both studies showed a 31% greater reduction from baseline in the frequency of agitation symptoms, was well-tolerated with a low incidence of discontinuations. The safety profile was consistent with the known safety profile of brexpiprazole in other indications
- Brexpiprazole was approved in the US for MDD and schizophrenia in adults & in Health Canada for the same indication while in Japan and EU for the treatment of schizophrenia
Veozah
Active ingredient: fezolinetant Approved: May 15, 2023
Company: Astellas Disease: Vasomotor Symptoms
- The US FDA has approved Veozah (45mg, qd) for the treatment of mod. to sev. vasomotor symptoms (VMS) due to menopause. The approval was based on the results from the (BRIGHT SKY) program incl. three P-III clinical trials as part of a development program in ~3,000 patients across the US, Canada, and EU
- In (SKYLIGHT 1 & 2) trials, results emphasized the safety and efficacy of fezolinetant for the treatment of VMS associated with menopause. while (SKYLIGHT 4) demonstrated the long-term safety of fezolinetant
- Additionally, the MAA for fezolinetant is also under regulatory review in the EU, Switzerland, and Australia
Miebo
Active ingredient: perfluorohexyloctane Approved: May 18, 2023
Company: Bausch + Lomb Disease: Dry Eye Disease
- The US FDA has approved Miebo (perfluorohexyloctane ophthalmic solution) for the treatment of the signs and symptoms of dry eye disease (DED). The therapy is expected to be commercially available in H2’23
- The approval was based on the P-III (GOBI) & (MOJAVE) trials evaluating Miebo in a ratio (1:1) in 1200 patients with a history of DED and clinical signs of Meibomian gland dysfunction, showed that Miebo met both primary sign and symptom efficacy EPs. The two 1EPs were changed from baseline at 8wk. in total corneal fluorescein staining (tCFS) and eye dryness Visual Analog Scale (VAS) score
- Patients experienced relief of symptoms as early as 15 days and through Day 57 with a reduction in VAS eye dryness score & tCFS favoring MIEBO
Rinvoq
Active ingredient: upadacitinib Approved: May 19, 2023
Company: AbbVie Disease: Crohn's Disease
- The approval was based on 3 P-III trials incl. (U-EXCEED & U-EXCEL) induction studies & (U-ENDURE) maintenance study to evaluate upadacitinib (45mg, qd) as IT & (15/30mg, qd) as MT vs PBO
- The results showed that more patients achieved the co-1EPs in both studies treated with Rinvoq 45mg @12wk. and (15 & 30mg) @52wks. demonstrated an endoscopic response (34% & 46% vs 3% & 13%) in 2 induction studies & (28% & 41% vs 7%) in the maintenance study; clinical remission (36% & 46% vs 18% & 23%) & (42% & 55% vs 14%), respectively
- In the 2EPs from the IT & MT, corticosteroid-free clinical remission (30% & 40% vs 11% & 13%) & (42% & 53% vs 14%), a greater proportion of patients achieved clinical response @2wk. The safety profile was consistent with the known safety profile of Rinvoq
Epkinly
Active ingredient: epcoritamab Approved: May 22, 2023
Company: AbbVie Disease: Diffuse Large B-Cell Lymphoma
- The US FDA has approved Epkinly for adult patients with r/r DLBCL. The therapy is being co-developed by AbbVie & Genmab
- The approval was based on the P-I/II trial (EPCORE NHL-1) evaluating epcoritamab (SC) in patients with r/r B-cell lymphoma. The results showed ORR (61%) with CR (38%) and PR (23%), and m-DoR was 15.6mos. after a median follow-up of 9.8mos., 82% of patients had disease refractory to the last therapy & 29% were refractory to CAR T-cell therapy. The safety profile was consistent with prior studies
- Epkinly, an IgG1-bispecific Ab developed using Genmab's DuoBody technology. Both companies will share commercial responsibilities in the US & Japan while AbbVie responsible for further global commercialization
Vyjuvek
Active ingredient: beremagene geperpavec Approved: May 22, 2023
Company: Krystal Biotech Disease: Dystrophic Epidermolysis Bullosa
- The approval was based on the 2 studies (GEM-1/2) & (GEM-3) trials evaluating Vyjuvek, the first-ever redosable gene therapy. The product is expected to be available in the US in Q3’23
- The P-III trial (GEM-3) results published in the NEJM demonstrated that the trial met its 1EPs of complete wound healing @6mos. and its 2EPs of complete wound healing @3mos. The therapy was well tolerated with no drug-related SAEs or discontinuations due to treatment-related events
- In the (GEM-1/2) trial, the results showed durable wound closure, expression of full-length COL7 in the skin, and anchoring fibril assembly with minimal reported AEs. The results were published in Nature Medicine
Opvee
Active ingredient: nalmefene Approved: May 22, 2023
Company: Indivior Disease: Opioid Overdose
- The US FDA has approved Opvee nasal spray for the emergency treatment of known or suspected opioid overdose induced by natural or synthetic opioids in adults and pediatric patients aged ≥12yrs. Opvee is expected to be available in Q4’23
- The approval was based on the PD study evaluating the effect of Opvee on remifentanil-induced respiratory depression incl. 61 opioid-experienced, non-dependent study patients
- The results showed a fast onset of reversal of respiratory depression b/w 2.5 and 5min. & full recovery was seen as early as 5min. after administrating Opvee. Opvee is supplied in a ready-to-use, unit-dose nasal spray device that delivers 2.7mg of nalmefene
Xacduro
Active ingredient: sulbactam-durlobactam Approved: May 23, 2023
Company: Innoviva Specialty Therapeutics Disease: Pneumonia
- The US FDA has approved Xacduro (IV) for hospital-acquired bacterial pneumonia & ventilator-associated bacterial pneumonia (HABP/VABP) caused by Acinetobacter in patients aged ≥18yrs.
- The approval was based on a P-III trial (ATTACK) evaluating Xacduro vs colistin which showed that Xacduro was found to be non-inferior over colistin for 1EPs of 28-day all-cause mortality in patients with carbapenem-resistant Acinetobacter inf. with a significant difference in clinical cure rates
- The therapy was well tolerated & exhibited a favorable safety profile across the clinical program. Xacduro is supplied as a kit containing a single-dose vial of sulbactam 1g & 2 single-dose vials of durlobactam (0.5g in each vial) & is expected to be available in 2023
Brixadi
Active ingredient: buprenorphine Approved: May 23, 2023
Company: Braeburn Disease: Opioid Use Disorder
- The US FDA has approved Brixadi extended-release inj. for SC use (CIII) to treat mod. to sev. opioid use disorder. Brixadi is available in 2 formulations: a weekly inj. for patients who have just started treatment & a monthly version who are already being treated with a transmucosal buprenorphine-containing product. The product is expected to be available in the US in Sept 2023
- The approval was based on a P-III trial evaluating Brixadi vs sublingual buprenorphine/naloxone in 428 patients which showed that Brixadi met the 1EPs of noninferiority for responder rate (16.9% vs 14.0%) & also met 2EPs of superiority in the percentage of negative opioid assessments from 4-24wk.
- Brixadi will be available through a restricted distribution program via the BRIXADI REMS program & is administered by a healthcare professional
Ayvakit
Active ingredient: avapritinib Approved: May 24, 2023
Company: Blueprint Medicines Disease: Indolent Systemic Mastocytosis
- The approval was based on the (PIONEER) trial evaluating Ayvakit (25mg, qd) + BSC vs PBO + BSC in 251 patients
- The results showed an improvement in the 1EPs & 2EPs incl. overall symptoms & measures of mast cell burden, was well-tolerated with a favorable safety profile, patients completed their treatment (96% vs 93%), AEs (90.8% vs 93%) & SAEs (5% vs 11.3%)
- The (PIONEER) trial results, incl. an OLE study showed the clinical benefits of Ayvakit @48wks., presented at AAAAI 2023. The therapy was approved in the US for 3 indications i.e., ISM, SM & unresectable or metastatic GIST harboring a PDGFRA exon 18 mutation & EU for ASM, SM-AHN or MCL & unresectable or metastatic GIST harboring the PDGFRA D842V mutation
16. Pfizer’s Paxlovid Receives the US FDA’s Approval for the Treatment of Severe COVID-19
Paxlovid
Active ingredient: nirmatrelvir and ritonavir Approved: May 25, 2023
Company: Pfizer Disease: COVID-19
- The US FDA has approved Paxlovid (nirmatrelvir & ritonavir tablets) for adults who are at high risk for progression to sev. COVID-19, incl. hospitalization or death
- The approval was based on the totality of scientific evidence evaluating Paxlovid incl. P-II/III study (EPIC-HR) results in unvaccinated, non-hospitalized adults aged ≥18yrs. which showed an 86% reduction in risk of COVID-19-related hospitalization or death from any cause through 28 Days who initiated treatment with Paxlovid within 5 days of symptoms onset
- The approval was also based on the 2EPs of the P-II/III study (EPIC-SR) which showed a numerical reduction in COVID-19-related hospitalizations or death from any cause through 28 Days in a sub-group of non-hospitalized adults
17. Lexicon’s Inpefa (sotagliflozin) Receives the US FDA’s Approval for the Treatment of Heart Failure
Inpefa
Active ingredient: sotagliflozin Approved: May 26, 2023
Company: Lexicon Disease: Heart Failure
- The US FDA has approved Inpefa to reduce the risk of cardiovascular death, hospitalization for HF, and urgent heart failure visits in adults with HF or type 2 diabetes mellitus, CKD, and other cardiovascular risk factors
- The approval was based on the 2 P-III studies (SOLOIST-WHF) & (SCORED) in ~12000 patients. The (SOLOIST-WHF) trial results showed a reduction in risk of the composite of hospitalizations for HF, urgent visits for HF & cardiovascular death by 33% over PBO who had been recently hospitalized for worsening HF
- No. of 1EPs events per 100 patient-years was 51.3 vs 76.4 in (SOLOIST-WHF) trial; 5.6 vs 7.5 in (SCORED). The therapy is expected to be commercially available in the US at the end of June 2023
Posluma
Active ingredient: flotufolastat F 18 Approved: May 30, 2023
Company: Blue Earth Diagnostics Disease: Prostate Cancer
- The US FDA has approved Posluma, an optimized, rh PSMA-targeted PET imaging agent in men with prostate cancer with suspected metastasis. Posluma is expected to be commercially available in the US in early June 2023
- The approval was based on the 2 P-III trials (LIGHTHOUSE & SPOTLIGHT) evaluating the safety and diagnostic performance of Posluma. The (LIGHTHOUSE) study results showed high specificity for the detection of pelvic lymph nodes over histopathology standard of truth in men with PSMA+ lesions before radical prostatectomy
- The (SPOTLIGHT) study demonstrated 83% detection rates even at low PSA levels in men with suspected prostate cancer recurrence based on elevated PSA following prior therapy
Related Post: Insights+: The US FDA New Drug Approvals in April 2023
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














